Efficacy & Safety Data
VYJUVEK demonstrated a significantly higher percentage of complete wound healing* (100% closure) compared with placebo1
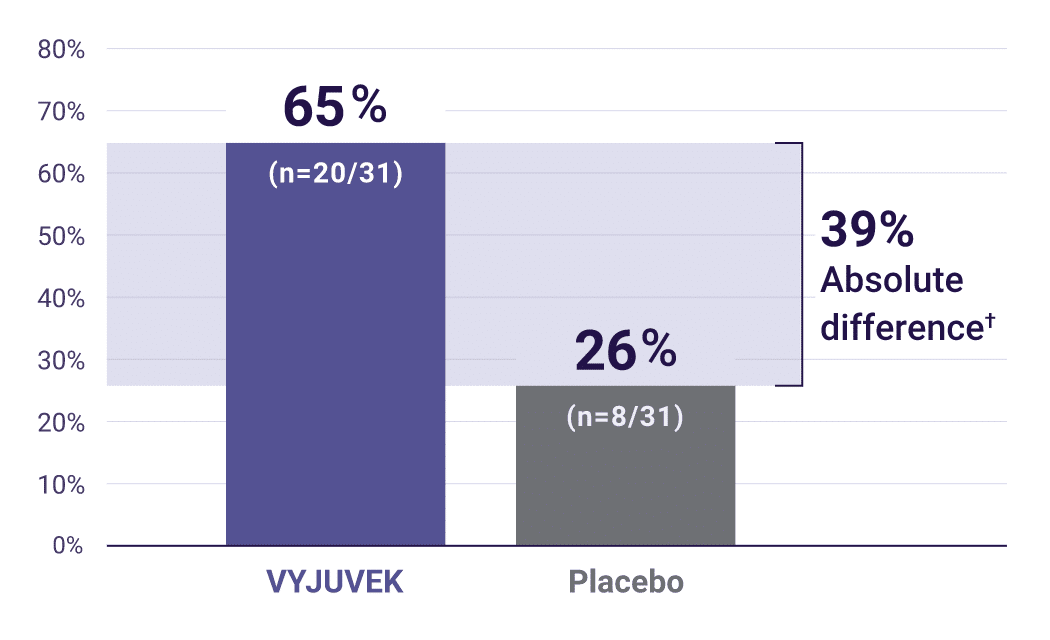
Primary endpoint: primary wounds with complete wound healing* (100% closure) at 6 months (N=31).1
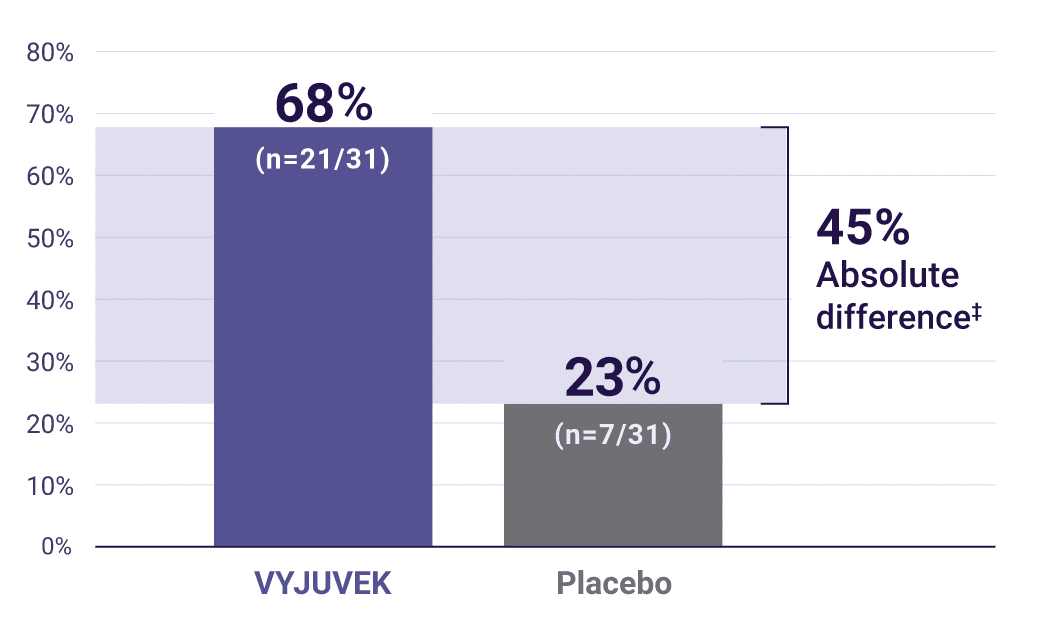
Key secondary endpoint: primary wounds with complete wound healing* (100% closure) at 3 months (N=31).1
*Complete (100%) wound closure was defined as durable wound closure (skin re-epithelialization without drainage) evaluated at two consecutive visits two weeks apart.1,2
†Primary endpoint: (95% CI: 14, 63)P=0.012.
‡Secondary endpoint: (95% CI: 22, 69)P=0.003.
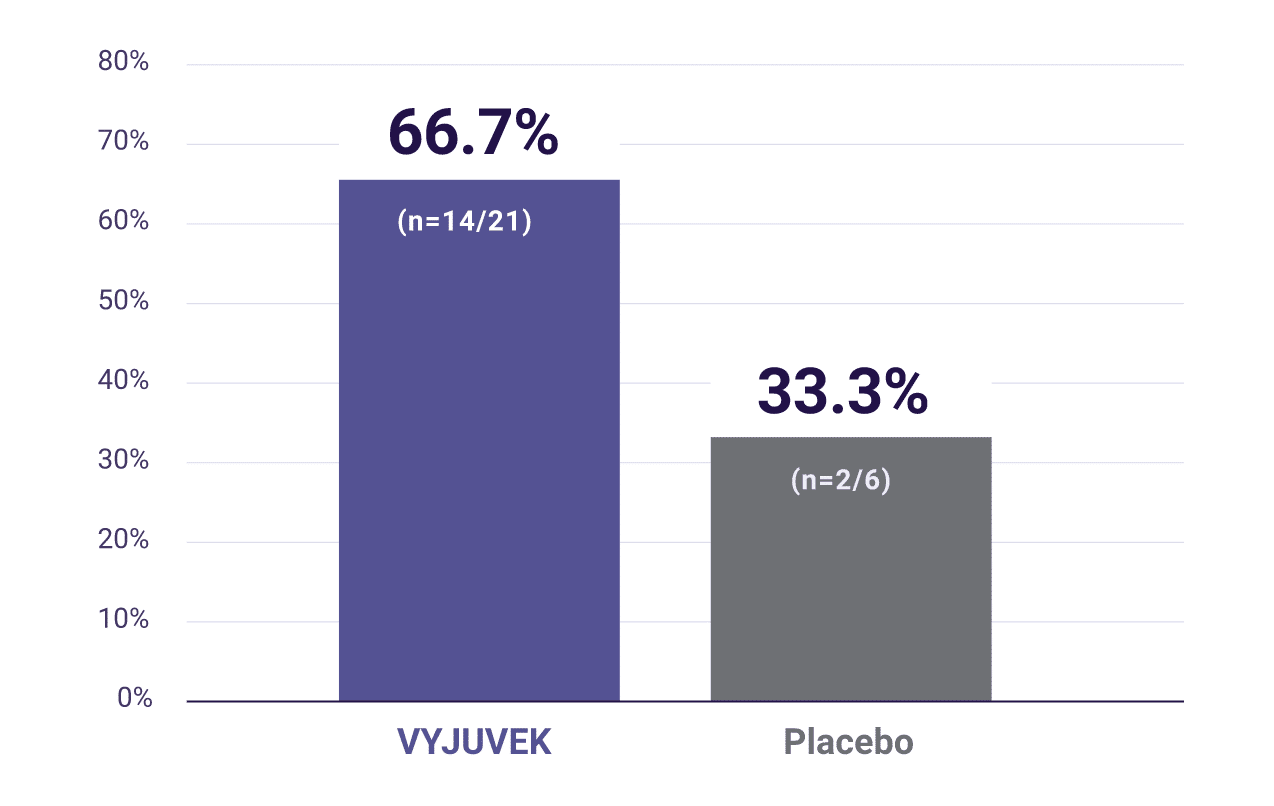
Of the wounds treated with VYJUVEK that were closed§ at
3 months, 67% (14/21) were also closed at 6 months.2
3 months, 67% (14/21) were also closed at 6 months.2
In a post-hoc analysis,|| wounds that were closed at 3 months—either VYJUx placebo-treated—were then re-evaluated at 6 months to determine what percentage of these wounds were closed.
These data are from a post-hoc exploratory analysis of both VYJUVEK-treated and placebo-treated wounds. The findings are based on a limited sample size and should be interpreted carefully.
Wounds closed at 3 months also closed at 6 months.
§Complete (100%) wound closure was defined as durable wound closure (skin re-epithelialization without drainage) evaluated at two consecutive visits two weeks apart.1,2
||Data on file.
BEFORE & AFTER IMAGES
- Across small, medium, and large wounds, VYJUVEK achieved complete wound closure
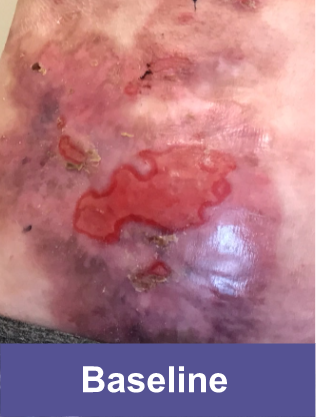
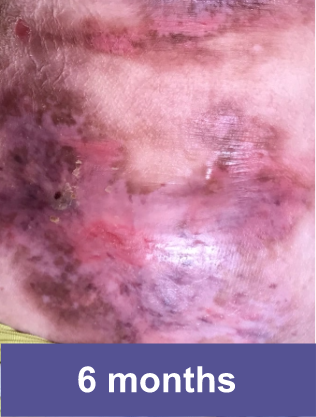
Patient A: Small Wound2
Pediatric female, lower abdomen
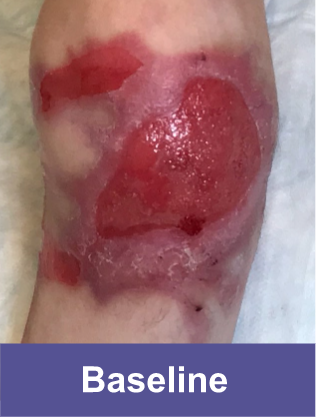
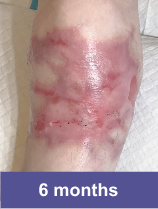
Patient B: Medium Wound2
Pediatric male, knee
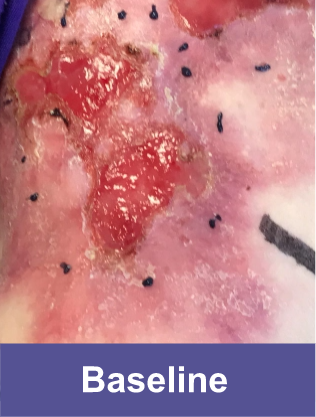
Patient C: Medium Wound2
Adult female, lower shoulder blade
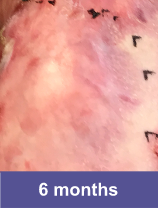
Patient D: Large Wound3
Adult male, back
Complete wound closure was achieved 2 months into the Open Label Extension trial following completion of the GEM-3 trial.¶ ¶The Open Label Extension trial was designed to assess long-term safety. Evaluation of this wound's complete wound closure was based on same method in GEM-3.
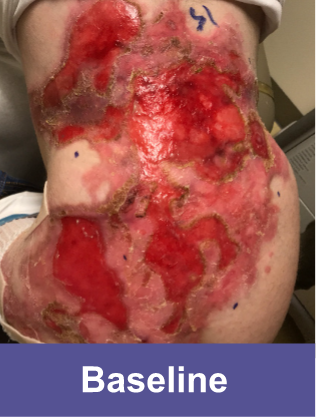
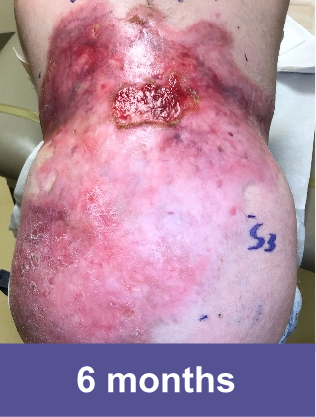
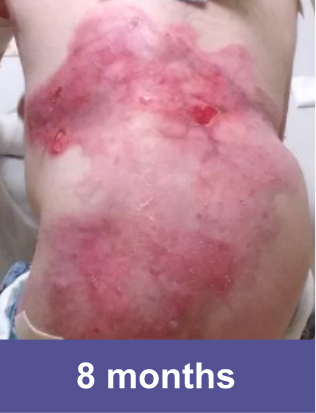
Hear about a patient's experience with VYJUVEK.
See Patient VideoVYJUVEK was well tolerated by patients1
- No discontinuation due to adverse reactions occurred
| Most Common Adverse Reactions (>5%) |
Patients, n (%) (N=31) |
|---|---|
| Itching | 3 (10) |
| Chills | 3 (10) |
| Redness | 2 (6) |
| Rash | 2 (6) |
| Cough | 2 (6) |
| Runny nose | 2 (6) |
