Efficacy & Safety Data
VYJUVEK demonstrated a significantly higher percentage of complete wound healing* (100% closure) compared with placebo1,2
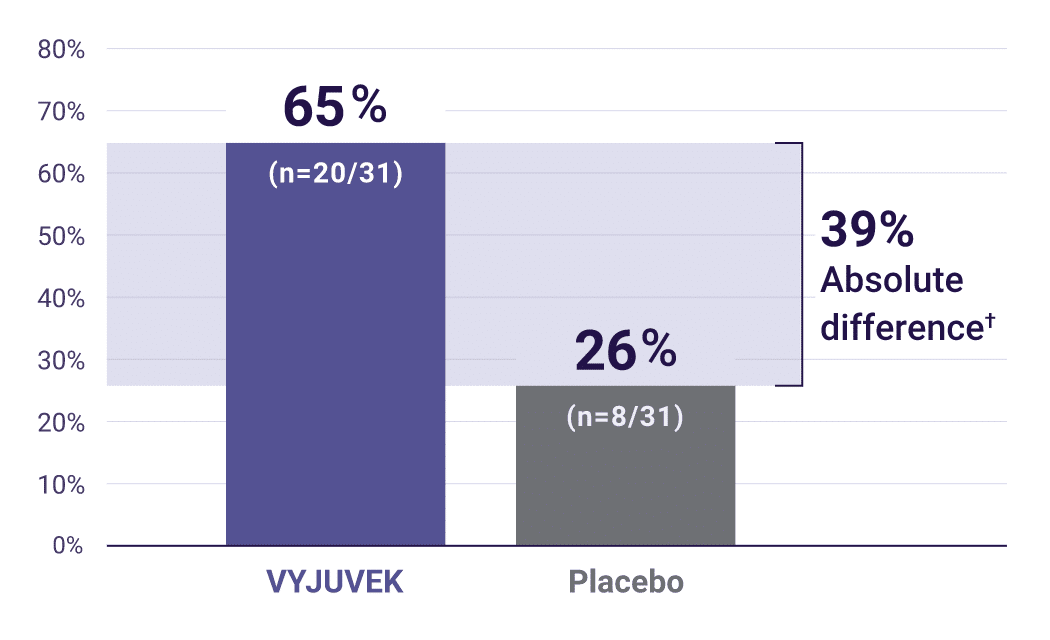
Primary endpoint: primary wounds with complete wound healing* (100% closure) at 6 months (N=31).1
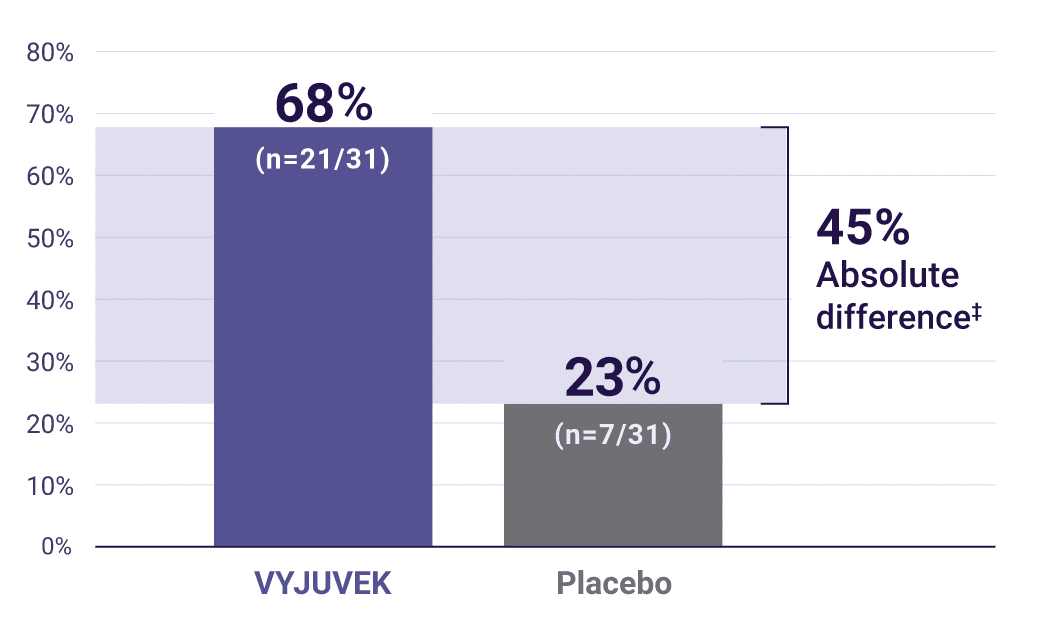
Key secondary endpoint: primary wounds with complete wound healing* (100% closure) at 3 months (N=31).1
*Complete (100%) wound closure was defined as durable wound closure (skin re-epithelialization without drainage) evaluated at 2 consecutive visits 2 weeks apart.2
†Primary endpoint: (95% CI: 14, 63)P=0.012.
‡Secondary endpoint: (95% CI: 22, 69)P=0.003.
Demonstrated durability: at both 3 and 6 months, complete wound healing was seen in 50% of wounds treated with VYJUVEK vs 7% of wounds treated with placebo2
DEB wounds by nature are dynamic and prone to reopening. The molecular correction provided by VYJUVEK was demonstrated in the reclosure of wounds in the OLE study.3
BEFORE & AFTER IMAGES
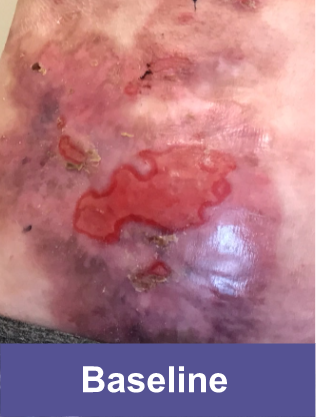
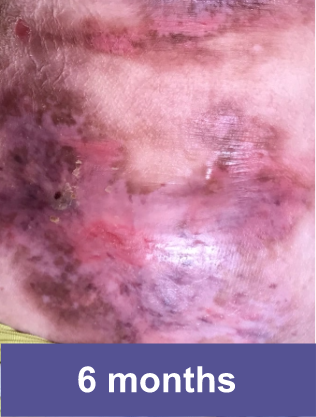
Patient A: Small Wound2,3
Pediatric female, lower abdomen
Patient B: Medium Wound2,3
Pediatric male, knee
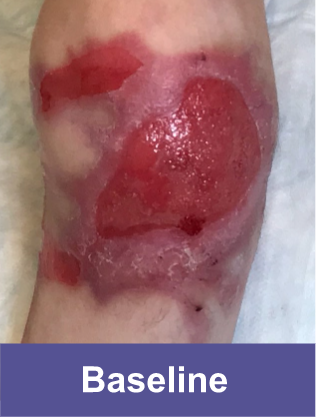
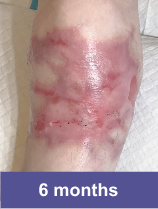
Patient C: Medium Wound2,3
Adult female, lower shoulder blade
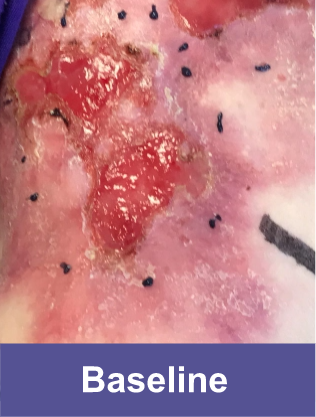
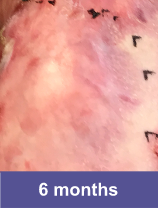
Patient D: Large Wound2,3
Adult male, back
Complete wound closure was achieved 2 months into the Open Label Extension (OLE) trial following completion of the GEM-3 trial.¶
¶The OLE trial was designed to assess long-term safety. Evaluation of this wound's complete wound closure was based on same method in GEM-3.3
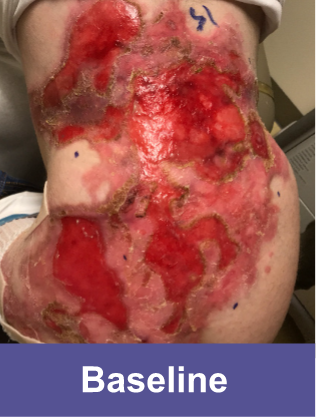
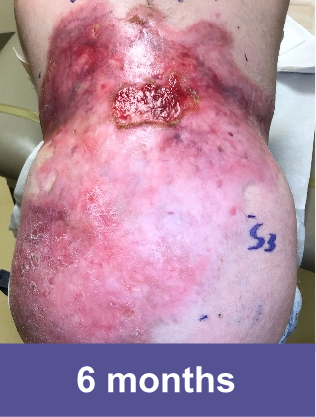
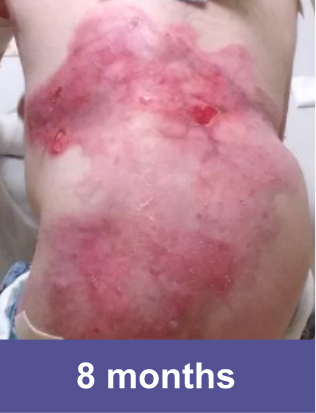
VYJUVEK clinical data demonstrates long-term efficacy and safety1-3
Phase 3 Baseline
End of GEM-3
End of OLE
VYJUVEK was well tolerated by patients1
- No discontinuation due to adverse reactions occurred1
| Most Common Adverse Reactions (>5%) |
Patients, n (%) (N=31) |
|---|---|
| Itching | 3 (10) |
| Chills | 3 (10) |
| Redness | 2 (6) |
| Rash | 2 (6) |
| Cough | 2 (6) |
| Runny nose | 2 (6) |
No new safety signals.3
Adverse events reported in the OLE study were similar to those observed in the GEM-3 study.
The majority of adverse events were mild or moderate in severity and consistent with those reported in patients with DEB.
See how to use VYJUVEK
Dosing & AdministrationVYJUVEK is a topical gel indicated for the treatment of wounds in adult and pediatric patients (from birth) with dystrophic epidermolysis bullosa (DEB) with mutation(s) in the collagen type VII alpha 1 chain (COL7A1) gene.
WARNINGS AND PRECAUTIONS
Accidental Exposure to VYJUVEK gel: VYJUVEK will not replicate in the subject’s cells and does not integrate into the subject cells’ native genetic material. For precautions, avoid direct contact with treated wounds (e.g., touching or scratching) and dressings of treated wounds until the next dressing change following treatment. Wear protective gloves when assisting patients with changing wound dressings and handling the disposal. In the event of accidental exposure (e.g., through a splash to the eyes or mucous membranes), flush with clean water for at least 15 minutes.
Clean all surfaces that may have come in contact with VYJUVEK biological suspension or gel and treat all spills with a virucidal agent.
Dispose all materials (e.g., vial, syringe, cleaning materials) that may have come in contact with VYJUVEK biological suspension or gel into a biohazard container.
ADVERSE REACTIONSThe most common adverse reactions (>5%) were itching, chills, redness, rash, cough, and runny nose.
- Please see Important Safety Information above and click here for full Prescribing Information.
1. VYJUVEK [prescribing information]. Krystal Biotech, Inc.; 2023.
2. Guide SV, Gonzalez ME, Bagci IS, et al. Trial of beremagene geperpavec (B-VEC) for dystrophic epidermolysis bullosa. N Engl J Med. 2022;387(24):2211-2219. doi:10.1056/NEJMoa2206663
3. Marinkovich MP, Paller AS, Guide SV, et al. Long-term safety and tolerability of beremagene geperpavec-svdt (B-VEC) in an open-label extension study of patients with dystrophic epidermolysis bullosa. Am J Clin Dermatol. Published online April 12, 2025. doi:10.1007/s40257-025-00942-y; https://doi.org/10.1007/s40257-025-00942-y
VYJUVEK is a topical gel indicated for the treatment of wounds in adult and pediatric patients (from birth) with dystrophic epidermolysis bullosa (DEB) with mutation(s) in the collagen type VII alpha 1 chain (COL7A1) gene.
WARNINGS AND PRECAUTIONS
Accidental Exposure to VYJUVEK gel: VYJUVEK will not replicate in the subject’s cells and does not integrate into the subject cells’ native genetic material. For precautions, avoid direct contact with treated wounds (e.g., touching or scratching) and dressings of treated wounds until the next dressing change following treatment. Wear protective gloves when assisting patients with changing wound dressings and handling the disposal. In the event of accidental exposure (e.g., through a splash to the eyes or mucous membranes), flush with clean water for at least 15 minutes.
Clean all surfaces that may have come in contact with VYJUVEK biological suspension or gel and treat all spills with a virucidal agent.
Dispose all materials (e.g., vial, syringe, cleaning materials) that may have come in contact with VYJUVEK biological suspension or gel into a biohazard container.
ADVERSE REACTIONSThe most common adverse reactions (>5%) were itching, chills, redness, rash, cough, and runny nose.
- Please see Important Safety Information above and click here for full Prescribing Information.
1. VYJUVEK [prescribing information]. Krystal Biotech, Inc.; 2023.
2. Guide SV, Gonzalez ME, Bagci IS, et al. Trial of beremagene geperpavec (B-VEC) for dystrophic epidermolysis bullosa. N Engl J Med. 2022;387(24):2211-2219. doi:10.1056/NEJMoa2206663
3. Marinkovich MP, Paller AS, Guide SV, et al. Long-term safety and tolerability of beremagene geperpavec-svdt (B-VEC) in an open-label extension study of patients with dystrophic epidermolysis bullosa. Am J Clin Dermatol. Published online April 12, 2025. doi:10.1007/s40257-025-00942-y; https://doi.org/10.1007/s40257-025-00942-y
You are about to leave the VYJUVEKHCP.com website
Are you sure you want to leave?
