Dosing & Administration
VYJUVEK is a noninvasive topical gel with a weekly maximum dose dependent upon patient age1
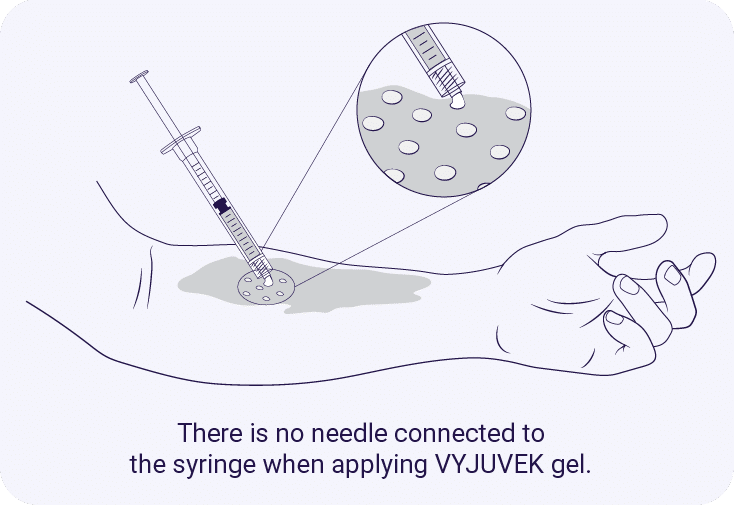
*Images are illustrative. Total surface area covered may vary. Follow the 1 cm × 1 cm application process in section 2.3 of the full Prescribing Information.
VYJUVEK is administered weekly by a healthcare professional in‑home or in‑office1
VYJUVEK gel is applied in small droplets that are evenly spaced (1 cm × 1 cm apart) on the selected wounds to form a thin film after a hydrophobic dressing is applied. Proceed to treat other wounds until the maximum weekly dose is reached.1
- Prior to application, VYJUVEK suspension must be mixed with excipient gel
- VYJUVEK gel should be applied weekly to skin wounds until they are closed
- The volume of VYJUVEK gel applied each week may vary, up to the maximum weekly dose, as wound size decreases or increases
- Prioritize weekly treatment to previously treated wounds until closure before starting treatment of new wounds
- Healthcare providers and close contacts should avoid direct contact with treated wounds and dressings for the first 24 hours following treatment
- Dispose all materials (e.g., vial, syringe, needle, cleaning materials) that may have come in contact with VYJUVEK biological suspension or gel into a biohazard bag or container
- In the event of an accidental exposure, flush with clean water for at least 15 minutes

It is important to ensure weekly treatments with
VYJUVEK until wounds close.
Watch these videos to learn how to prepare and administer VYJUVEK
Get your patients started on VYJUVEK with Krystal Connect™ patient support
Get StartedVYJUVEK is a topical gel indicated for the treatment of wounds in patients 6 months of age and older with dystrophic epidermolysis bullosa (DEB) with mutation(s) in the collagen type VII (COL7A1) gene.
WARNINGS AND PRECAUTIONS
Accidental Exposure to VYJUVEK gel: VYJUVEK will not replicate in the subject’s cells and does not integrate into the subject cells’ native genetic material. For precautions, avoid direct contact with treated wounds (e.g., touching or scratching) and dressings of treated wounds for approximately 24 hours following treatment. Wear protective gloves when assisting subjects with changing wound dressings and handling the disposal. In the event of accidental exposure (e.g., through a splash to the eyes or mucous membranes), flush with clean water for at least 15 minutes.
Clean all surfaces that may have come in contact with VYJUVEK biological suspension or gel and treat all spills with a virucidal agent.
Dispose all materials (e.g., vial, syringe, needle, cleaning materials) that may have come in contact with VYJUVEK biological suspension or gel into a biohazard bag or container.
ADVERSE REACTIONSThe most common adverse reactions (>5%) were itching, chills, redness, rash, cough, and runny nose.
- Please see Important Safety Information above and click here for full Prescribing Information.
1. VYJUVEK [prescribing information]. Krystal Biotech, Inc.; 2023.
VYJUVEK is a topical gel indicated for the treatment of wounds in patients 6 months of age and older with dystrophic epidermolysis bullosa (DEB) with mutation(s) in the collagen type VII (COL7A1) gene.
WARNINGS AND PRECAUTIONS
Accidental Exposure to VYJUVEK gel: VYJUVEK will not replicate in the subject’s cells and does not integrate into the subject cells’ native genetic material. For precautions, avoid direct contact with treated wounds (e.g., touching or scratching) and dressings of treated wounds for approximately 24 hours following treatment. Wear protective gloves when assisting subjects with changing wound dressings and handling the disposal. In the event of accidental exposure (e.g., through a splash to the eyes or mucous membranes), flush with clean water for at least 15 minutes.
Clean all surfaces that may have come in contact with VYJUVEK biological suspension or gel and treat all spills with a virucidal agent.
Dispose all materials (e.g., vial, syringe, needle, cleaning materials) that may have come in contact with VYJUVEK biological suspension or gel into a biohazard bag or container.
ADVERSE REACTIONSThe most common adverse reactions (>5%) were itching, chills, redness, rash, cough, and runny nose.
- Please see Important Safety Information above and click here for full Prescribing Information.
1. VYJUVEK [prescribing information]. Krystal Biotech, Inc.; 2023.
For US Healthcare Professionals Only
This website is intended for US healthcare professionals.
Would you like to continue?
